Bulletin N°6




The briefs of bulletin N°6
Article N°1
Dimitrios Papadopoulos and al.
Accelerated Cellular Senescence in Progressive Multiple Sclerosis: A Histopathological Study
Annals of Neurology – February 2025
Article N°2
Oligodendrocytes in Alzheimer’s disease pathophysiology
Nature Neuroscience – January 2025
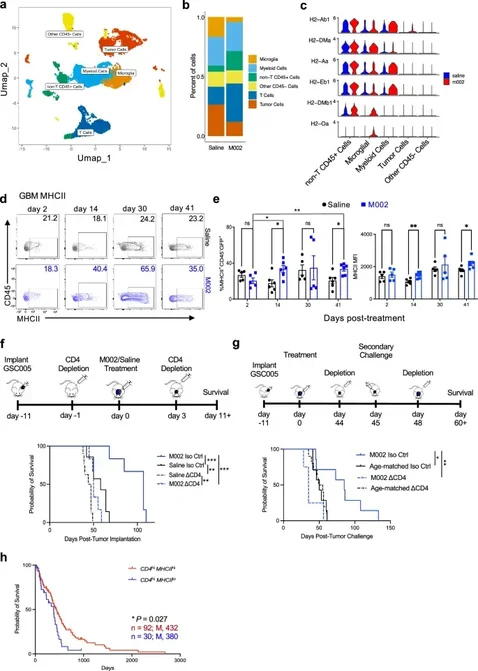
Article N°3
Vaccine-induced T cell receptor T cell therapy targeting a glioblastoma stemness antigen
Nature Communications - February 2025
Article N°4
Gemistocytic tumor cells programmed for glial scarring characterize T cell confinement in IDH-mutant astrocytoma
Nature Communications – January 2025