Bulletin N°2
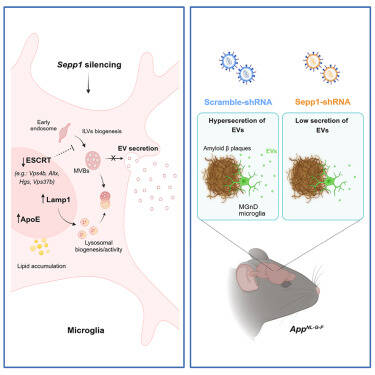




The briefs of bulletin N°2
Article N°1
Elevated expression of the retrotransposon LINE-1 drives Alzheimer’s disease-associated microglial dysfunction.
Acta Neuropathologica – November 2024
Article N°2
Haploinsufficiency at the CX3CR1 locus of hematopoietic stem cells favors the appearance of microglia-like cells in the central nervous system of transplant recipients.
Nature Communications – November 2024
Article N°3
Review Article - Current and Future Roles of Chimeric Antigen Receptor T-Cell Therapy in Neurology.
JAMA Neurology – November 2024
Article N°4
Review Article - Current state and perspectives of CAR T cell therapy in central nervous system diseases.
Brain – November 2024